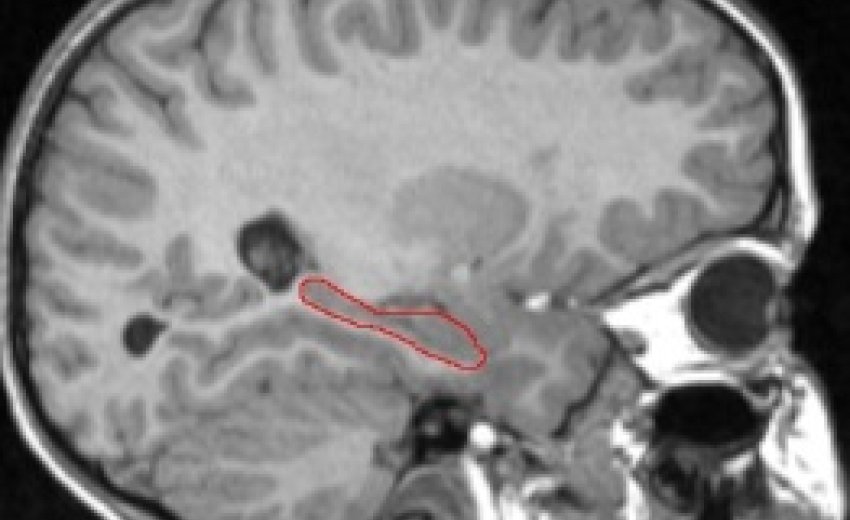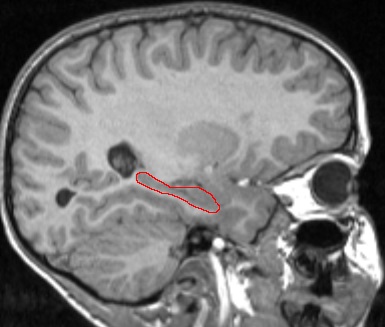
Hippocampus Outlined in Red (T1-weighted MRI image)
We are currently examining whether the effect of hypoglycemic episodes is visible in brain regions associated with memory, especially the hippocampus (see picture below). The hippocampus is a cashew-shaped gray matter structure known to play a role in the "consolidation" of memory, or the conversion of short term into long term.
Many complications of diabetes, including kidney disease, foot problems and vision problems are generally well recognized. But the disease’s impact on the brain is often overlooked.
For the past five years, a team led by Beth Israel Deaconess Medical Center (BIDMC) neurophysiologist Vera Novak, MD, PhD, has been studying the effects of diabetes on cognitive health in older individuals and has determined that memory loss, depression and other types of cognitive impairment are a serious consequence of this widespread disease.
Now, Novak’s team has identified a key mechanism behind this course of events. In a study published in the November 2011 issue of the journal Diabetes Care, they report that in older patients with diabetes, two adhesion molecules – sVCAM and sICAM – cause inflammation in the brain, triggering a series of events that affect blood vessels and, eventually, cause brain tissue to atrophy. Importantly, they found that the gray matter in the brain’s frontal and temporal regions — responsible for such critical functions as decision-making, language, verbal memory and complex tasks – is the area most affected by these events.
“In our previous work, we had found that patients with diabetes had significantly more brain atrophy than did a control group,” explains Novak, Director of the Syncope and Falls in the Elderly (SAFE) Program in the Division of Gerontology at BIDMC and Associate Professor of Medicine at Harvard Medical School. “In fact, at the age of 65, the average person’s brain shrinks about one percent a year, but in a diabetic patient, brain volume can be lowered by as much as 15 percent.”
Diabetes develops when glucose builds up in the blood instead of entering the body’s cells to be used as energy. Known as hyperglycemia, this condition often goes hand-in-hand with inflammation. Novak wanted to determine if chronic inflammation of the blood vessels was causing altered blood flow to the brain in patients with diabetes.
To test this hypothesis, Novak’s team recruited 147 study subjects, averaging 65 years of age. Seventy one of the subjects had type 2 diabetes and had been taking medication to manage their conditions for at least five years. The other 76 were age and sex-matched non-diabetic controls.
Study subjects underwent a series of cognitive tests, balance tests and standard blood-pressure and blood-glucose tests. Serum samples were also collected to measure adhesion molecules and several other markers of systemic inflammation. To determine perfusion (blood flow) measures in the brain, patients also underwent functional MRI testing, in which a specialized imaging technique known as arterial spin labeling (developed by BIDMC MR physicist David Alsop, PhD) was used in conjunction with a standard MRI to measure vascular reactivity in several brain regions and to show changes in blood flow.
As predicted, the scans showed that the diabetic patients not only had greater blood vessel constriction than the control subjects, but they also had more atrophied brain tissue, particularly gray matter. The results also showed that, in the patients with diabetes, the frontal, temporal and parietal regions of the brain were most affected. Similarly, the team’s measurements of serum markers confirmed that high glucose levels were strongly correlated with higher levels of inflammatory cytokines.
“It appears that chronic hyperglycemia and insulin resistance – the hallmarks of diabetes – trigger the release of adhesion molecules [sVCAM and sICAM] and set off a cascade of events leading to the development of chronic inflammation,” says Novak. “Once chronic inflammation sets in, blood vessels constrict, blood flow is reduced, and brain tissue is damaged. ”
This discovery now provides two biomarkers of altered vascular reactivity in the brain. “If these markers can be identified before the brain is damaged, we can take steps to try and intervene,” says Novak, explaining that some data indicates that medications may improve vascoreactivity.
But more important, she says, the new findings provide still more reason for doctors and patients to focus greater attention on the management – and prevention – of diabetes.
“Cognitive decline affects a person’s ability to successfully complete even the simplest of everyday tasks, such as walking, talking or writing,” says Novak. “There are currently 25.8 million cases of type 2 diabetes in the United States alone, which is more than eight percent of our total population. The effects of diabetes on the brain have been grossly neglected, and, as our findings confirm, are issues that need to be addressed.” ###
This study was supported, in part, by grants from the National Institute of Aging, the National Institute of Diabetes, Digestive and Kidney Diseases, the American Diabetes Association, and the National Center for Research Resources.
Study coauthors include BIDMC investigators Peng Zhao, PhD, Brad Manor, PhD, Ervin Sejdic, PhD, David Alsop, PhD, and Medha Munshi, MD; Amir Abduljalil, PhD, of Ohio State University; Paula Roberson, PhD, of the University of Arkansas for Medical Sciences; and Peter Novak, MD, PhD, of the University of Massachusetts Medical School.
Beth Israel Deaconess Medical Center is a patient care, teaching and research affiliate of Harvard Medical School and ranks third in National Institutes of Health funding among independent hospitals nationwide. BIDMC is a clinical partner of the Joslin Diabetes Center and a research partner of the Dana-Farber/Harvard Cancer Center.